7 rows Calcium chloride dihydrate USP 990-1070. 500 grams Calcium Chloride to mol 450515 mol.
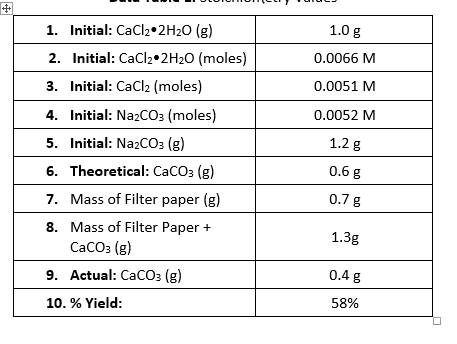
Solved 1 Initial Cacl2 2h2o G 2 Initial Cacl2 2h20 Chegg Com
1 grams Calcium Chloride to mol 000901 mol.
. CaCl22H2O is a white deliquescent compound at room temperature. Computed by PubChem 21 PubChem release 20210507 Monoisotopic Mass. Molar mass of CaCl₂ 2 H₂O atomic weight of Ca 1 atomic weight of Cl 2 atomic weight of H 4 atomic weight of O 2.
The molecular weight for Calcium Chloride Dihydrate CaCl22H20 is 2H2O. It has several common applications such as brine for refrigeration plants ice and dust control on roads and in cement. 1 Introduction To Chemistry And Introduction To Active Learning 2 Matter And Energy 3 Measurement And Chemical Calculations 4 Introduction To Gases 5 Atomic Theory.
3 rows Calcium Chloride molecular weight. Calcium chloride dihydrate weighs 185 gram per cubic centimeter or 1 850 kilogram per cubic meter ie. Calcium chloride is a salt that can be obtained from natural brines as a by-product from synthetic soda ash production and can be produced from hydrochloric acid and limestone.
50 grams Calcium Chloride to mol 045052 mol. Calcium chloride dihydrate weighs 185 gram per cubic centimeter or 1 850 kilogram per cubic meter ie. How many moles are in the substance.
Which example has 121024 hydrogen atoms. You will have 11098 g mol-1 2 18015 g mol- 14701 g mol-1. A substance has twice the number of particles as 12 grams of carbon-12.
200 grams Calcium Chloride to mol 180206 mol. 258gmol 25809843 gmol. Mass of 64gmol 25652 gmol.
It is a salt that is solid at room temperature and it behaves as a typical ionic halide. 10 grams Calcium Chloride to mol 00901 mol. Calcium chloride dihydrate is an inorganic hydrated compound which has the chemical formula CaCl 2 H 2 O 2.
What is Calcium Chloride Dihydrate. The molar mass of this compound is 11156 gmol. Be sure to include the mass of all elements in the formula including the elements in water.
Its melting point is 176 C 3488 F density 185 gcm3. Density of calcium chloride dihydrate is equal to 1 850 kgm³. What is the molar mass of calcium chloride dihydrate CaCl2 2H2O.
Correct answer to the question 30 points what is the molar mass of calcium chloride dihydrate cacl2 2h2o. Calcium chloride dihydrate 20 percent by weight dissolved in ethanol 95 percent ABV has been used as a sterilant for male animals. Be sure to include the mass of all elements in the formula including the elements in water.
Calcium chloride dihydrateA few things to consider when finding the molar mass for CaCl2 2H2O-. 100 grams Calcium Chloride to mol 090103 mol. Density of calcium chloride dihydrate is equal to 1 850 kgm³.
Computed by Cactvs 34611 PubChem release 20190618 Heavy Atom Count. An Active Learning Approach. When considering the structure of this compound it has one calcium chloride molecule in association with two water molecules.
Computed by PubChem 21 PubChem release 20210507 Topological Polar Surface Area. Mass of 11gmol 26981538 gmol. What is calcium chloride dihydrate used for.
Calcium chloride dehydrate CaCl₂ 2 H₂O have a molar mass equal to 147 gmol. It is soluble in water. In Imperial or US customary measurement system the density is equal to 115492 pound per cubic foot lbft³ or 1069 ounce per cubic inch ozinch³.
Browse Calcium chloride dihydrate and related products at MilliporeSigma. Explanation of how to find the molar mass of CaCl2 2H2O. Molar mass of CaCl2 110984 gmol.
Since the molar mass of Calcium is 4008 g and the molar mass of Chlorine is 3545 it would be 4008 g 3545 g 3545 g with 2 Chlorines according to the subscript That leaves you with 11098 g. 1000 grams Calcium Chloride to mol 901031 mol. All three methods are in use with the synthetic route being used for the largest part of the volume.
About Calcium chloride dihydrate. Now in order to find the molar mass of the hydrate use the molar mass of anhydrous calcium chloride and the molar mass of water. To calculate the molar mass of calcium chloride dehydrate CaCl₂ 2 H₂O we use the following formula.
Calcium chloride meets USP testing. 11098 gmol 1 Appearance White powder hygroscopic. The molar mass of a compound is just the molar masses of every element in the compound added up.
Calcium chloride is an inorganic compound. Mass of 147gmol 1469149 gmol. Be sure to include the mass of all elements in the formula including the elements in water.
The solution is injected into the testes of the animal. 1 mole of water H2O What is the molar mass of calcium chloride dihydrate CaCl2 2H2O.
Molar Mass Molecular Weight Of Cacl2 2h2o Calcium Chloride Dihydrate Youtube

Molar Mass Molecular Weight Of Cacl2 2h2o Calcium Chloride Dihydrate Youtube

Molar Mass Molecular Weight Of Cacl2 Youtube

What Is The Molar Mass Of Calcium Chloride Dihydrate Cacl2 2h2o Be Sure To Include The Mass Of Brainly Com
0 Comments